Abstract
The current standard of care to prevent graft versus host disease (GVHD) after HLA mismatched unrelated donor (URD) allogeneic hematopoietic cell transplantation (HCT) is tacrolimus, methotrexate (MTX) and anti-thymocyte globulin (ATG). Recently, an approach based on administration of post-transplant cyclophosphamide (PT-Cy) demonstrated promise in a prospective trial for HLA mismatched URD HCT. Here, we compared tacrolimus/MTX/ATG (ATG group) treatment, with that of PT-Cy, mycophenolate mofetil, and tacrolimus or sirolimus (PT-Cy group) after HLA mismatched URD HCT, at two academic centers with similar peri-transplant supportive care practices.
Subjects that underwent HCT from a URD mismatched at one or more loci among HLA-A, -B, -C, and -DRB1 from 2010-2020 were included. The primary endpoint of our study was one-year GVHD-free, relapse-free survival (GRFS). Of 128 total subjects included (ATG: 46; PT-Cy: 82), the median age was 54.7 (range: 21.0 - 72.0) in the ATG group and 60.3 (21.0 - 75.2) in the PT-Cy group. Notably, 74 subjects (57.8%) belonged to a racial/ethnic minority, while 53 (41.4%) received a bone marrow (BM) graft, and 26 (20.3%) received a highly mismatched (i.e., <6/8 HLA) graft. A higher proportion of PT-Cy subjects received BM (50% vs. 26.1%, P= 0.01) and highly mismatched grafts (30.5% vs. 2.2%, P=0.001). The groups were well matched for key demographic data, including indication for HCT, high-risk disease, and HCT comorbidity index.
The rate of primary neutrophil engraftment at day 28 post HCT was similar between the two groups: 91% (83%-99%), in the ATG group, and 90% (84%-97%), in the PT-Cy group. The median day of neutrophil engraftment was +17 in the ATG, and +20 in the PT-Cy groups. Platelet engraftment at day 100 was 84.8% (74.4% - 95.2%) and 86.6% (79.2% - 94%) in the ATG and PT-Cy groups, respectively. Among subjects that survived 3-months post HCT and had donor chimerism testing, full donor chimerism (≥95% donor) was achieved in 24/26 (92.3%) patients in the ATG group, and 61/68 (89.7%) subjects in the PT-Cy group.
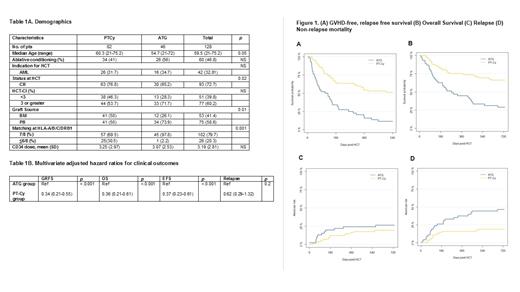
Overall, the one-year GRFS was 16% (95% confidence interval: 8% - 31%) vs. 54% (44% - 66%, p < 0.001) in the ATG and PT-Cy groups, respectively. The adjusted, multivariate hazard ratio (HR) for GRFS was 0.34 (0.21 - 0.55, p < 0.001), in association with the use of PT-Cy. The one-year overall survival (OS) in the ATG group was 45% (32% - 62%) vs. 75% (66% - 85%, p < 0.001) in the PT-Cy group. There was no difference in 2-year relapse incidence (27%, 14-39% vs. 19%, 10% - 28%, p = 0.2), whereas 1-year non-relapse mortality (NRM) was increased with ATG-based prophylaxis: (38%, 23% - 52% vs. 16%, 9% - 25%, p = <0.001). The use of PT-Cy was associated with improved GRFS for patients treated with BM (HR = 0.27, 0.11 - 0.62; p = 0.002) and G-CSF mobilized, peripheral blood derived (PB) grafts (HR = 0.39, 0.21 - 0.71; p = 0.002). BM grafts were not an independent predictor of GRFS (HR: 1.2, 0.8 - 1.9; p = 0.5) in a multivariate Cox regression model.
The use of PT-Cy, compared to ATG, was associated with lower rates of grade 3-4 acute GVHD in the entire cohort (ATG: 22%, 18% - 45% vs. PT-Cy: 15%, 8% - 23%; p = 0.03), and in the sub-cohorts of patients transplanted with PB (ATG: 33%, 17% - 49% vs. PT-Cy: 20%, 9% - 33%) and BM grafts (ATG: 25%, 1% - 50% vs. PT-Cy: 10%, 1% - 19%). Chronic GVHD requiring systemic immune suppression was more frequent in the ATG/PB group (26%, 13% - 42%) than in the ATG/BM (8%, 0% - 24%), the PT-Cy/PB (8%, 2% - 19%), and the PT-Cy/BM (10%, 1% - 19%) groups.
Despite greater HLA-mismatching, PT-Cy following mismatched URD HCT resulted in superior GRFS, OS and lower NRM and GVHD rates compared to ATG-based GVHD prophylaxis. These results indicate that PT-Cy is the preferred method to prevent GVHD after mismatched URD HCT and confers a benefit in patients treated with either PB or BM-derived allografts. Combinatorial approaches, using the T-cell co-stimulatory blockading agent abatacept, in addition to tacrolimus/methotrexate, may also prevent GVHD in this population, and can be compared to a PT-Cy-based approach in a prospective clinical trial.
Jimenez: AbbVie: Research Funding; Takeda: Research Funding. Perales: MorphoSys: Honoraria; Bristol-Myers Squibb: Honoraria; Celgene: Honoraria; Karyopharm: Honoraria; Merck: Honoraria; Equilium: Honoraria; Miltenyi Biotec: Honoraria, Other; Medigene: Honoraria; Kite/Gilead: Honoraria, Other; Incyte: Honoraria, Other; Cidara: Honoraria; Takeda: Honoraria; Nektar Therapeutics: Honoraria, Other; Servier: Honoraria; Sellas Life Sciences: Honoraria; Omeros: Honoraria; NexImmune: Honoraria; Novartis: Honoraria, Other. Sauter: Precision Biosciences: Consultancy; Genmab: Consultancy; Celgene: Consultancy, Research Funding; Kite/Gilead: Consultancy; Bristol-Myers Squibb: Research Funding; GSK: Consultancy; Gamida Cell: Consultancy; Novartis: Consultancy; Spectrum Pharmaceuticals: Consultancy; Juno Therapeutics: Consultancy, Research Funding; Sanofi-Genzyme: Consultancy, Research Funding. Beitinjaneh: Kite/Gilead: Other: Ad Board Event Attendee. Ponce: CareDx: Consultancy, Honoraria; Ceramedix: Consultancy, Honoraria; Seres Therapeutics: Consultancy, Research Funding; Kadmon pharmaceuticals: Consultancy, Honoraria; Takeda Pharmaceuticals: Research Funding; Generon Pharmaceuticals: Consultancy.
Off-label use of Cyclophosphamide for Graft-versus-Host Disease prevention will be discussed in the abstract.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal